Revolutionizing Infection Detection


Discover our mission and vision in transforming diagnostics.
Watch CEO’s Message on Transforming Healthcare

Our Cutting-Edge Diagnostic
01.
RNA-Based Blood Test
Groundbreaking tests enhancing early disease diagnosis and treatment efficiency.
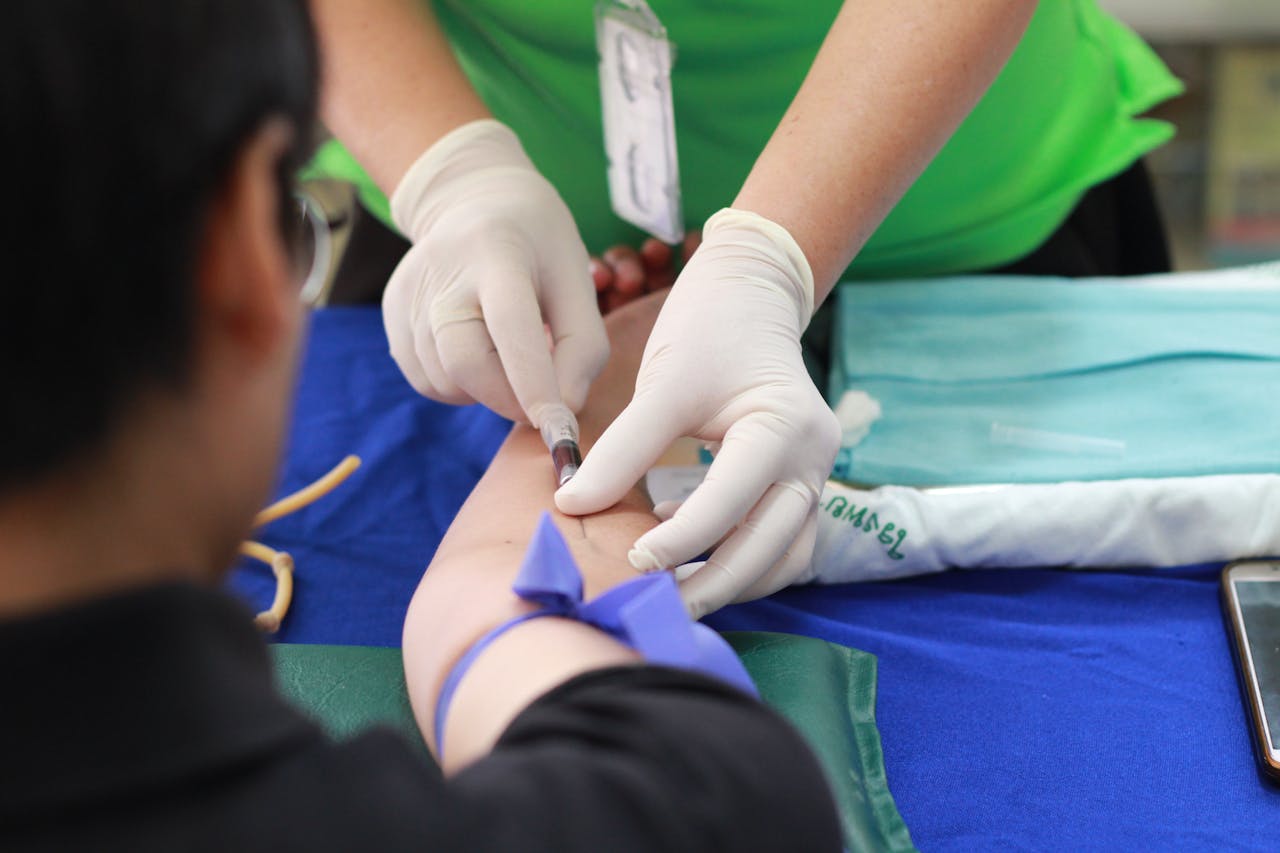
02.
Biomarker Panels Development
Innovative panels designed for precise infection diagnosis.
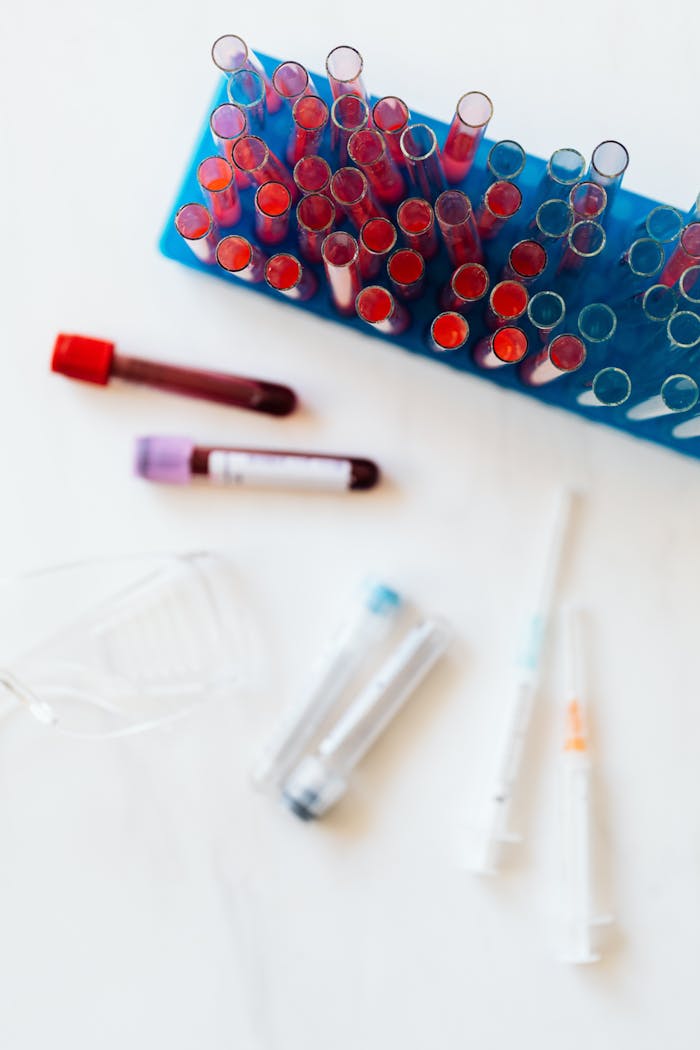
03.
Early infection Detection
Services focused on improving early detection accuracy to minimize treatment costs.

Why Choose Us
Innovative Solutions
We focus on breakthrough RNA-based technologies diagnose infections which will help optimize treatment strategies for better patient care.
Expert Collaboration
Partnering with leading research institutions, we bring cutting-edge scientific advancements directly to clinical applications.
Cost-Effective Testing
Our tests are designed to reduce costs through early detection, making advanced diagnostics more accessible for all patients.


