Our Company
True Bearing has diagnostic biomarker panels for cardiovascular disease, appendicitis, and pneumonia/respiratory infections. With unique technology for biomarker discovery, True Bearing can address the enormous market for precision personalized human disease diagnostics and identify new drug targets. These biomarker panels were developed in The Division of Genomic Medicine at The George Washington University by Dr. Tim McCaffrey, in close collaboration with clinical colleagues.
The discovery process used the latest single molecule RNA sequencing techniques, resulting in tests are that are accurate and cost effective.
Our Lead Products
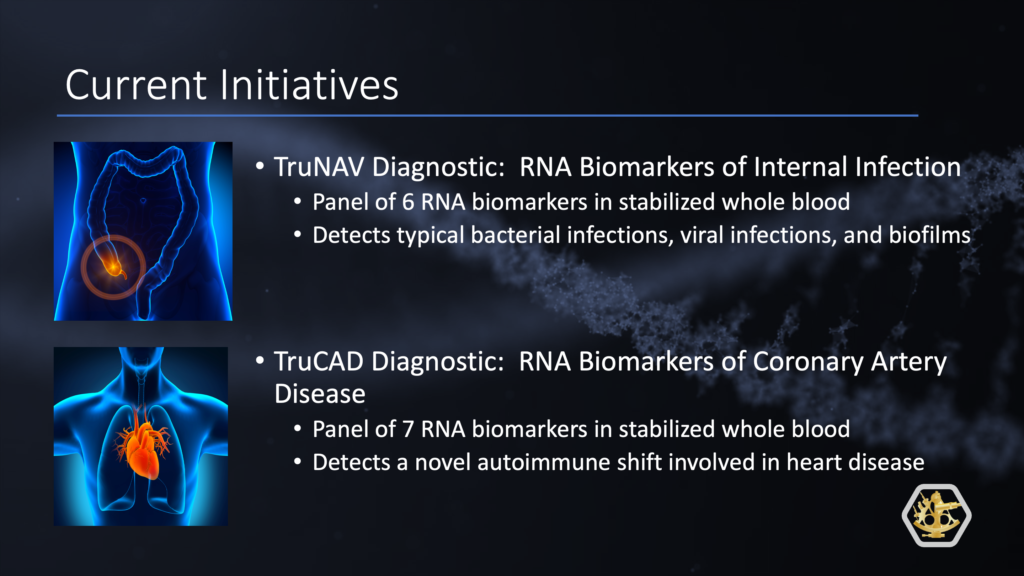
TruNAV is a novel blood RNA-based diagnostic test designed for the early detection of internal infections. TruNAV is based on detecting RNA transcripts that are highly sensitive to Neutrophil ActiVation by any pathogen, making it an ideal test for the early detection of infections. TruNAV is unique because it can recognize different types of infections: typical infections such as pneumonia, viral infections like COVID19, and importantly, biofilm-type infections that occur in appendicitis, diabetic foot ulcers, and prosthetic joint infections.
TruCAD is a “liquid angiogram” that senses immune imbalances in blood with great accuracy. TruCAD is the first in a collection of comprehensive RNA-based diagnostics to guide precision care. Cardiovascular disease is the largest healthcare market in the US, especially coronary artery disease (CAD), but it is largely neglected by genomic advances. There are more than 600,000 deaths a year from cardiovascular diseases in the U.S., costing more than $500 billion in total annual expense.
